All Living Things Need Water
 In some organisms, (like jellyfish!) up to 90 percent of their body weight comes from water.
In some organisms, (like jellyfish!) up to 90 percent of their body weight comes from water.- Up to 60 percent of the human body is water.
- The brain is composed of 70 percent water!
- Blood is 82 percent water.
- The lungs are nearly 90 percent water.
Just How Much Water is There?
 The total water supply of planet earth is 326 million cubic miles (a cubic mile is an imaginary cube (a square box) measuring one mile on each side). A cubic mile of water equals more than one trillion gallons.
The total water supply of planet earth is 326 million cubic miles (a cubic mile is an imaginary cube (a square box) measuring one mile on each side). A cubic mile of water equals more than one trillion gallons.- About 3,100 cubic miles of water, mostly in the form of water vapor, is in the atmosphere at any one time. If it all fell as precipitation at once, the Earth would be covered with only about 1 inch of water.
- Each day, 280 cubic miles of water evaporate or transpire into the atmosphere.
- Of the freshwater on Earth, much more is stored in the ground than is available in lakes and rivers. More than 2,000,000 cubic miles of fresh water is stored in the Earth, most within one-half mile of the surface.
- Contrast that with the 60,000 cubic miles of water stored as fresh water in lakes, inland seas and rivers.
- But if you really want to find fresh water, the most is stored in the 7,000,000 cubic miles of water found in glaciers and icecaps, mainly in the polar regions and in Greenland.
The Chemical Composition of Water
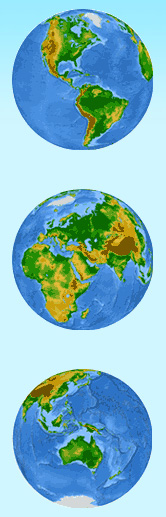
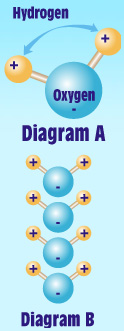 You probably know water’s chemical description is H2O. As the diagram [A] shows, that is one atom of oxygen bound to two atoms of hydrogen. The hydrogen atoms are “attached” to one side of the oxygen atom, resulting in a water molecule having a positive charge on the side where the hydrogen atoms are, and a negative charge on the other side, where the oxygen atom is. Since opposite electrical charges attract, water molecules tend to attract each other, making water kind of “sticky.”
You probably know water’s chemical description is H2O. As the diagram [A] shows, that is one atom of oxygen bound to two atoms of hydrogen. The hydrogen atoms are “attached” to one side of the oxygen atom, resulting in a water molecule having a positive charge on the side where the hydrogen atoms are, and a negative charge on the other side, where the oxygen atom is. Since opposite electrical charges attract, water molecules tend to attract each other, making water kind of “sticky.”
As diagram [B] shows, the side with the hydrogen atoms (positive charge) attracts the oxygen side (negative charge) of a different water molecule. (If the water molecule here looks familiar, remember that everyone's favorite mouse is mostly water, too!)
Why is the Ocean Salty?
 If you get into folk stories and mythology, you will see that almost every culture has a story explaining how the oceans became salty. The answer is really very simple. Salt in the ocean comes from rocks on land. Here’s how it works:
If you get into folk stories and mythology, you will see that almost every culture has a story explaining how the oceans became salty. The answer is really very simple. Salt in the ocean comes from rocks on land. Here’s how it works:
The rain that falls on the land contains some dissolved carbon dioxide from the surrounding air. This causes the rainwater to be slightly acidic due to carbonic acid (which forms from carbon dioxide and water). The rain erodes the rock and the acid breaks down the rocks and carries it along in a dissolved state as ions. The ions in the runoff are carried to the streams and rivers to the ocean. Many of the dissolved ions are used by organisms in the ocean and are removed from the water. Others are not used up and are left for long periods of time where their concentrations increase over time.
The two ions that are present most often in seawater are chloride and sodium. These two make up over 90 percent of all dissolved ions in seawater. By the way, the concentration of salt in seawater (salinity) is about 35 parts per thousand. In other words, about 35 of 1,000 (3.5 percent) of the weight of seawater comes from the dissolved salts.